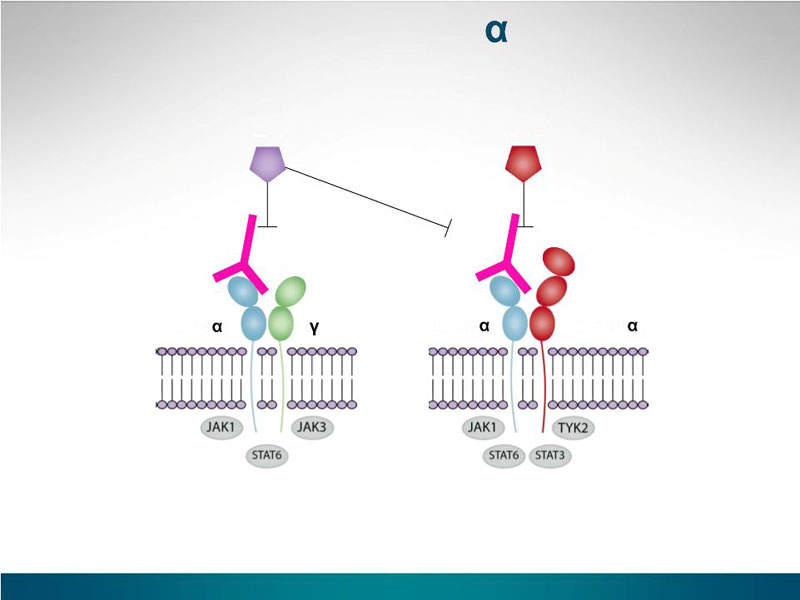
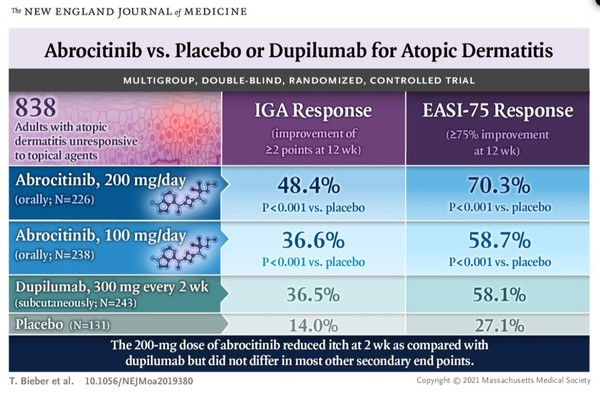
Efficacy and safety of dupilumab for the treatment of adult atopic dermatitis: A meta- analysis of randomized clinical trials Yue Han, MD, Yuxin Chen, - ppt download

Modify treatment for atopic dermatitis when patient response to dupilumab is partial or non-durable | SpringerLink

Dupilumab Improves Asthma and Sinonasal Outcomes in Adults with Moderate to Severe Atopic Dermatitis - The Journal of Allergy and Clinical Immunology: In Practice

Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial - The Lancet

Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo‐controlled, randomized phase III

Real-world persistence with dupilumab among adults with atopic dermatitis - Annals of Allergy, Asthma & Immunology
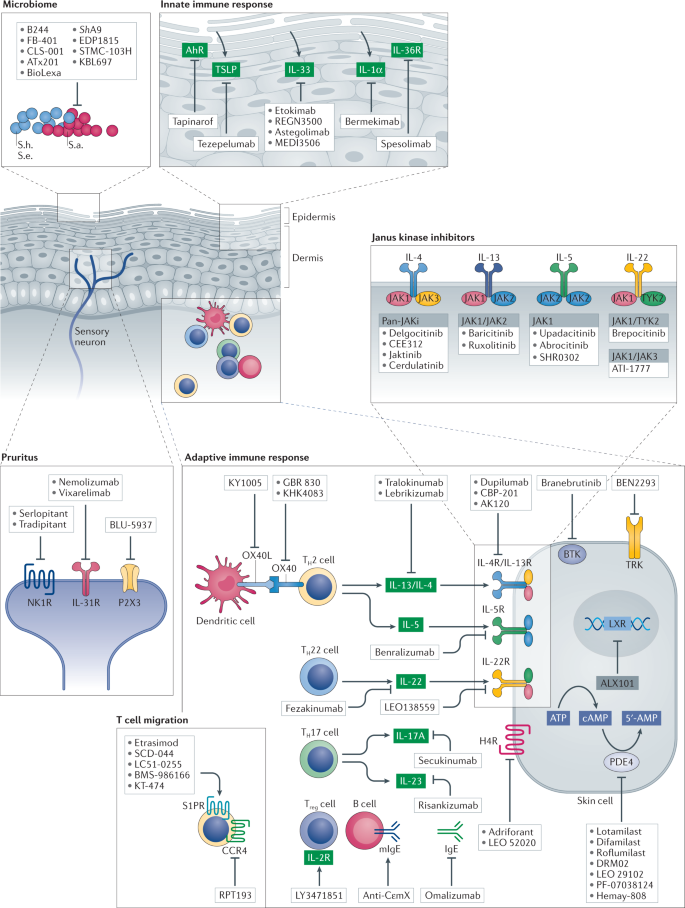
Atopic dermatitis: an expanding therapeutic pipeline for a complex disease | Nature Reviews Drug Discovery

Example of case before and after treatment with dupilumab 300mg every... | Download Scientific Diagram

Efficacy of House Dust Mite Sublingual Immunotherapy in Patients with Atopic Dermatitis: A Randomized, Double-Blind, Placebo-Controlled Trial - The Journal of Allergy and Clinical Immunology: In Practice

Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial - Journal of the American

Long-term follow-up and treatment outcomes of conjunctivitis during dupilumab treatment in patients with moderate-to-severe atopic dermatitis - The Journal of Allergy and Clinical Immunology: In Practice